The fragmentation of molecular ions into an assortment of fragment ions is a mixed blessing. The nature of the fragments often provides a clue to the molecular structure, but if the molecular ion has a lifetime of less than a few microseconds it will not survive long enough to be observed. Without a molecular ion peak as a reference, the difficulty of interpreting a mass spectrum increases markedly. Fortunately, most organic compounds give mass spectra that include a molecular ion, and those that do not often respond successfully to the use of milder ionization conditions.
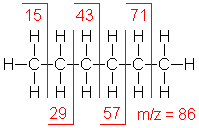
Shown below is the spectrum of hexane (another example of straight chain hydrocarbon) and the masses of the different fragments produced.

Spectra of branched saturated hydrocarbons are generally similar to those of straight-chain compounds, but the smooth curve of decreasing intensities is broken by the preferred fragmentation at each branch. This discontinuety is due to the formation of stable secondary and tertiary carbocations. For this reason, the molecular ion is much less abundant than for straight-chain alkanes. The most important mode of fragmentation in branched alkanes usually occurs at the branch point. In the mass spectrum of isobutane shown below the reduced intensity of the molecular ion peak (m/e 58). The scheme below shows the mechanism of fragmentation of isobutane.

A saturated ring hydrocarbon increases the relative intensity of the molecular ion peak and favor cleavage of the bond connecting the ring to the rest of the molecule. The fragmentation patterns of cycloalkanes may show mass clusters in a homologous series, as for the alkanes due to the presence of a straight chain hydrocarbon side chain.
Thus the fragmentation of cycloalkanes is charecterized by the loss of ethylene from the parent molecule or from intermediate radical-ions. Additionally, if the cycloalkane has a side chain, loss of that side chain is also a favorable mode of fragmentation. Scheme 5 provides the mechanism for the fragmentation for cyclohexane. The mass spectrum of cyclohexane has an abundant ion of m/e 56 arising from the loss of ethylene.

The mass apectra of three different saturated hydrocarbons are displayed below. Two are isomeric hexanes and the third is cyclohexane.
The following three examples are hydrocarbons having no functional groups.
Hexane shows the same fragmentation pattern as other unbranched alkanes. Thus, alkyl carbocations at m/e=15, 29, 43 and 57 amu provide the dominant peaks in the spectrum. The m/e=57 butyl cation (M-29) is the base peak, and the m/e=43 and 29 ions are also abundant.
Chain branching clearly influences the fragmentation of this isomeric hexane. The molecular ion at m/e=86 is weaker than that for hexane itself and the M-15 ion at m/e=71 is stronger. The m/e=57 ion is almost absent (try to find a simple cleavage that gives a butyl group). An isopropyl cation (m/e=43) is very strong, and the corresponding propene radical-cation at m/e=42 (colored orange), produced by loss of propane, gives the base peak.
In cyclohexane the fragmentation is profoundly changed. The molecular ion at m/e=84 is much stronger than the corresponding ions in acyclic compounds. The base peak at m/e=56 is produced by loss of ethene(CH2=CH2). The alkenyl cations at m/e=41 & 27 are stronger than the corresponding alkyl cations (m/e=43 & 29). The loss of methyl (m/e=69), and a corresponding small m/e=15 ion obviously require some hydrogen rearrangements.

fragmentation of 2,3-dimethylbutane
2-Olefins:
The molecular ion peak of olefins , espicially polyolefins is usually distinct. Location of the double bond in acyclic olefins is difficult because its facile migration in the fragment. In cyclic (especially polycyclic) olefins, location of the double bond is fequently evident as a result of the tendency for allylic cleavage without much double bond migration. Conjugation with a carbonyl group also fixes the position of the double bond. As with saturated hydrocarbons, cyclic olefins are characterized by clusters of peaks at intervals of 14 units.
3-Aromatic compounds:
Molecular ion peaks are strong due to the stable structure. The molecular ion peak is usually sufficiently large to allow accurate measurment of M+1 and M+2 peaks.
The fragmentation of the aromatic nucleus is somewhat complex, generating a series of peaks having m/e = 77, 65, 63, etc. While these peaks are difficult to describe in simple terms, they do form a pattern (the "aromatic cluster") that becomes recognizable with experience. If the molecule contains a benzyl unit, the major cleavage will be to generate the
 benzyl carbocation, which rearranges to form the tropylium ion (a prominent peak and often is the base peak at m/e 91). The molecular ion, again, represents loss of an electron and the peaks above the molecular ion are due to isotopic abundance. The base peak in toluene is due to loss of a hydrogen atom to form the relatively stable benzyl cation. This is thought to undergo rearrangement to form the very stable tropylium cation, and this strong peak at m/e = 91 is ery characterstic of compounds containing a benzyl unit. The frequently observed peak at m/e = 65 is due to the loss of neutral acetylene from the tropylium ion and the minor peaks below this arise from more complex fragmentation.
benzyl carbocation, which rearranges to form the tropylium ion (a prominent peak and often is the base peak at m/e 91). The molecular ion, again, represents loss of an electron and the peaks above the molecular ion are due to isotopic abundance. The base peak in toluene is due to loss of a hydrogen atom to form the relatively stable benzyl cation. This is thought to undergo rearrangement to form the very stable tropylium cation, and this strong peak at m/e = 91 is ery characterstic of compounds containing a benzyl unit. The frequently observed peak at m/e = 65 is due to the loss of neutral acetylene from the tropylium ion and the minor peaks below this arise from more complex fragmentation.
Alkylated polyphenyls and alkulated polycyclic aromatic hydrocarbons exhibit the same beta-cleavage as alkyl benzenes. The spectrum of naphthaline is shown below. The molecular ion is the bas peak due to stabilization of the formed cation.

4-Aldehydes and ketones:
Major pfragmentation peaks of aliphatic carbonyls result from the cleavage of the C-C bons adjacent to the carboxyl group results in the loss of hydrogen (molecular ion less 1) or the loss of CHO (molecular ion less 29).
Shown below are three example of aliphatic carbonyl compounds *Pentanal shosws a a typical fragmentation pattern of alkyl hydrocarbon chain (e.g. m/e=57, 43, 41, 29 & 27). The molecular ion at m/e=86 is very weak, as is the M-1 ion at m/e=85 . The m/e=29 ion is due to the beta-cleavage charecteristic of aldehydes and ketons forming HCO cation as well as the ethyl fragment due to the fragmentation of the side chain.
*Pentanal shosws a a typical fragmentation pattern of alkyl hydrocarbon chain (e.g. m/e=57, 43, 41, 29 & 27). The molecular ion at m/e=86 is very weak, as is the M-1 ion at m/e=85 . The m/e=29 ion is due to the beta-cleavage charecteristic of aldehydes and ketons forming HCO cation as well as the ethyl fragment due to the fragmentation of the side chain.
Rearrangement ions are observed at m/z=58 (possibly loss of CO) & 44 (rearrangement involving loss of propene).
*The molecular ion at m/e=86 is more abundant than in the previous aldehyde spectrum. The directive effect of the carbonyl group on fragmentation is also apparent here. Loss of carbonyl substituents by alpha-fragmentation include: loss of methyl (M-15 at m/e=71) and loss of a propyl radical (M-43 at m/z=43). These comprise the most prominent fragment ions.
*molecular ion at m/e=86 is about as strong as in 2-pentanone. Because of the symmetry, ethyl groups are the only carbonyl substituents that can be lost by an alpha-fr agmentation. The strongest fragment ions are found at m/e=57 (loss of an ethyl group) and m/e=29 (an ethyl cation).
The moelcular ion peak of aromatic ketones is prominent.Cleavage of aryl alkyl ketones occurs at the bond beta to the ring leaving a characterstic ArC=O+fragment which usually accounts for the base peak. Loss of CO from this fragment ion gives the aryl ion (m/e 77 in the case of acetophenones). If the alkyl chain is three carbons or longer, possibility of McLaferty rearrangement exists
3-Alcohol and sulphides:
The molecular ion peak of alcohol is small or non-existent. Cleavage of the C-C bond next to the oxygen usually occurs. Thus primary alcohol show of a prominent peak due to CH2=OH+.(m/e 31).Secondary and tertiary alcohols cleave analogously to give a prominent peak due to the fragment (m/e 45, 59, 73, etc.) and
(m/e 45, 59, 73, etc.) and  (m/e 59, 73, 87, etc.) , respectively. The
(m/e 59, 73, 87, etc.) , respectively. The
largest substituent is expelled readily.
Primary alcohols, in addition to the principle C-C cleavage nest to the oxygen atom, shows a homologous series of peaks of progressively decreasing intensity resulting from cleavage at C-C bonds successively removed from the oxygen giving a pattern simmilar to that produced by long chain hydrocarbons.
A distinct and sometimes prominent peak can usually be found at M-18 due to loss of water.
M-18 is frequently exaggerated by thermal decomposition of higher alcohols on hot interfaces.

Elemination of water together with elemination of an olefin from primary alcohols, accounts for the presence of a peak at M-(olefin+H2O), that is a peak at M-46, M-74, M-102,....
A peak at M-31 (see above) is quite diagnostic for a primary alcohol provided it is more intense than peaks at m/e 45, 59, etc.. In 1-pentanol the hydroxyl group is at the end of the five-carbon chain. There are two significant fragment ions, one at m/e=70 (loss of water), and the other at m/e=42 (loss of water and ethene). The fragment ion at m/e=55 is probably due to a methyl radical loss from the m/e=70 ion. The m/z=31 is due to CH2=OH+.
In 1-pentanol the hydroxyl group is at the end of the five-carbon chain. There are two significant fragment ions, one at m/e=70 (loss of water), and the other at m/e=42 (loss of water and ethene). The fragment ion at m/e=55 is probably due to a methyl radical loss from the m/e=70 ion. The m/z=31 is due to CH2=OH+.
3-Pentanol shows three significant fragment ions. Fragment due to Alpha-cleavage (loss of an ethyl radical) forms the m/e=59 base peak. Loss of water from this gives a m/e=41 fragment, and loss of ethene from m/e=59 gives a m/z=31 fragment
The molecular ion (m/e=90) is strong, and the presence of sulfur is indicated by a larger than usual M+2 (m/e=92) peak. This is true for the m/e=75 & 47 peaks as well. Loss of methyl and ethyl radicals generate ions at m/e=75 & 61. The odd-electron rearrangement ion at m/e=62 results from loss of ethene. Finally, m/e=47 may be formed from m/e=62 by loss of a methyl radical.
4-Amines:
The molecular ion is of low abundance or not detectable. When observable, its odd mass (when an odd number of nitrogens is present) is a good indication of the presence of an amine (nitrogen rule). Important fragments arise from cleavage of the carbon-carbon or carbon-hydrogen bond adjacent to the nitrogen (alpha-cleavage).
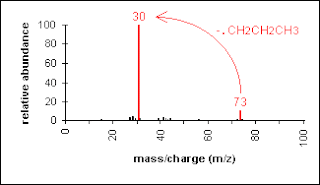
Another example is a secondary amine shown below. Again, the molecular ion peak is an odd number. The base peak is from the C-C cleavage adjacent to the C-N bond.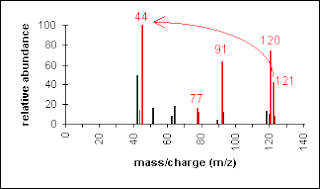

4-Amides:
The molecular ion is usually observable, and will be a good indication of the presence of an amide (the nitrogen rule is applicable). An important fragmentation pattern involves alpha-cleavage (breaking either bond to the carbonyl carbon). Primary amides show base peak due to mcklaferty rearrangement as shown below.

5-Esters:
Ester Fragments appear due to bond cleavage next to C=O (alkoxy group loss, -OR) and hydrogen rearrangements.
 The molecular ion in the mass spectrum of ethyl acetate is
The molecular ion in the mass spectrum of ethyl acetate isrelatively weak. The base peak results from an alpha-fragmentation of ethoxyl radical to give an m/e=43 ion. The loss of water to generate the at m/e=70 takes place. Small methyl and ethyl ions are found at m/e=15 & 29 due formation of methyl and ethyl cation fragments respectively.
The methyl propanoate spectrum , shows a more abundant molecular ion than ethyl acetate. The alpha fragmentation of methoxyl radical generates the strong m/e=57 ion. Alpha-fragmentation of the ethyl group leads to both m/e=59 & 29. A smaller methyl peak is also seen.
The spectrum of dimethylformamide (DMF) shows an abundant of its molecular ion (m/e=73) indicating its stability(the base peak). A small M-15 peak is observed, but the second most abundant ion is loss of HCO due to alpha-cleavage (m/z=44). Other ions at m/z=42, 30 & 28 are probably derived from the m/z=44 ion.
7-Carboxylic Acids:
The molecular ion is often of low abundance for carboxylic acids, but generally observable. As for all other carbonyl compounds, alpha-cleavage, beta-cleavage, and McLafferty rearrangements are the main fragmentation pattrns. The loss of hydroxyl radical (leading to an M - 17 ion) is indicative of the presence of the carboxylic acid functionality.
Thus in short chain acids, peaks due to the loss of OH (M-17) and COOH (M-45) are prominent due to cleavage of bonds next to C=O.


Ethers:
The molecular ion is usually of low abundance, but of higher abundance than the molecular ions of alcohols. Important fragments arise from cleavage of the carbon-oxygen bondand cleavage of the carbon-carbon bond adjacent to the oxygen (alpha-cleavage).

8-Halides:
As has ben discussed in details in lecture two, the presence of chlorine or bromine atoms is usually recognizable from isotopic peaks. On the other hand, the presence of Iodine is detected by the loss of 127 while the presence of flourine is observed by the loss of 19 (F+) or 20 (HF) amu.


